Aiming to improve the chances of all those affected.
Zugang zum PanTum Detect® Portal (nur für Krebs-Scan Kunden):

SIMPLE
A normal blood sample once a year is all it takes.
EARLY
Detection of tumor indicators in early, symptom-free stages.
GROUNDBREAKING
Prerequisite for targeted use of medical imaging (e.g. PET/CT, MRI).
ONE FOR ALLMOST ALL
A multi-cancer blood test for indications of almost all types of cancer.
NEED FOR EARLY CANCER DETECTION
In the majority of cases, cancer grows undetected for many years. The earlier it is detected, the better the chances of a complete recovery.
EVERY 2ND PERSON
DEVELOPS CANCER
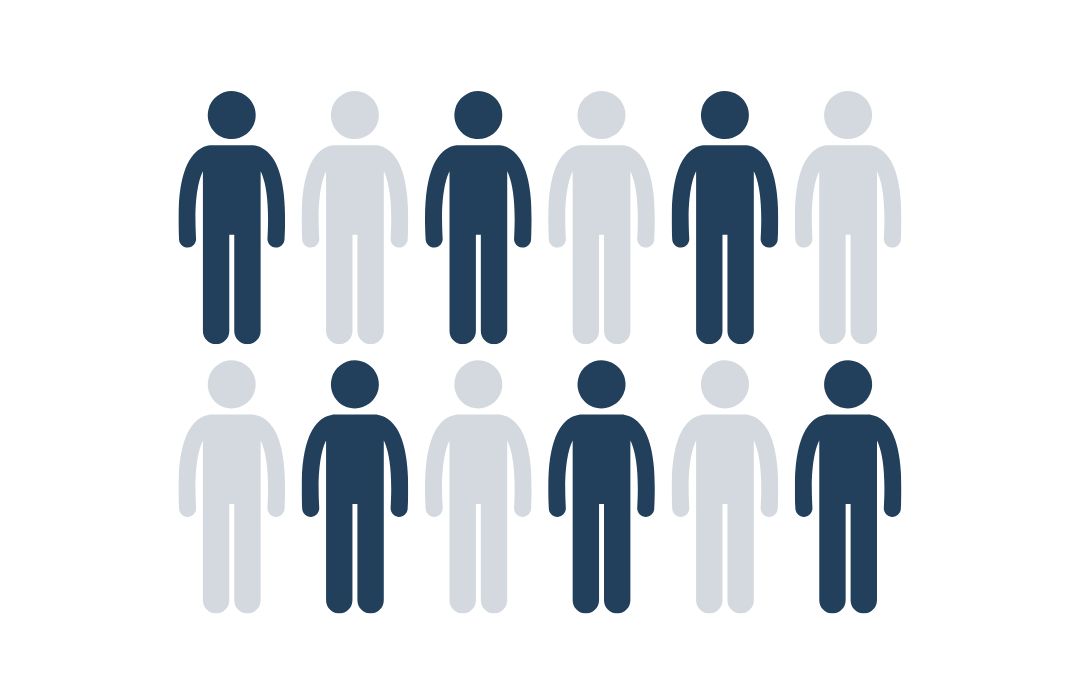
Statistically, about every other person develops cancer once in his or her life.
Source: Cancer Research UK
CANCER-SPECIFIC
SCREENING
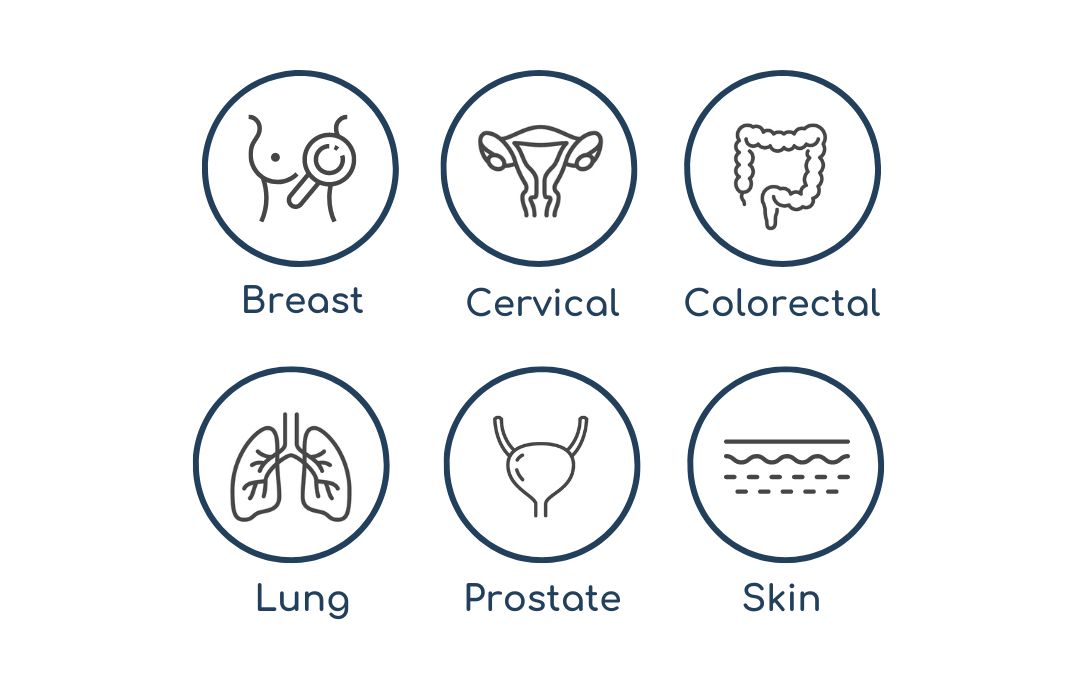
Regular cancer-specific screening examinations exist in selected countries for breast, cervical, colorectal, lung, prostate, and skin cancer.
Source: German Cancer Research Center; American Cancer Society
SCREENING GAP
CASE: GERMANY
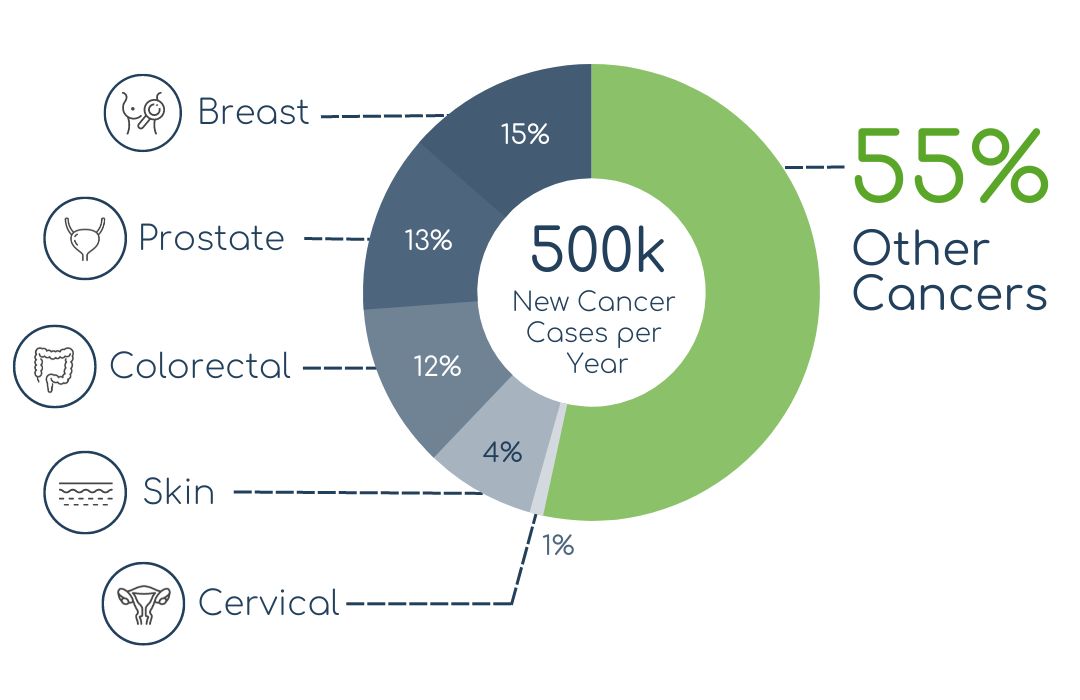
Even in a medically advanced country such as Germany, for 55% of the 500k annual new cancer cases, no regular screening examinations exist. That amounts to more than 250k cases every year.
Source: average yearly new cancer cases based on data of RKI (krebsdaten.de |2009-2018)
PANTUM DETECT® – DEVELOPED TO MAKE A DIFFERENCE
- Can give early indications for potential tumors, at a time when treatment options are manifold and chances of cure are high.
- Developed to detect indications at the right time and stage – not too early, not too late.
- Using a single blood sample once a year.
- Not cancer type specific: Works for almost all types of cancer.
- Detects signals for two biophyscial mechanisms that all types of cancer have in common.
- PanTum Detect® does not diagnose cancer or identify types of cancer, it gives general indications that need to be confirmed and localized in follow-up diagnostics, such as imaging procedures.
WHO IS IT FOR?
For anyone, who …
To see whether cancer screening makes sense for you individually given your current personal situation (age, family medical history, personal medical history, personal medical condition, etc.), please consult your physician.
WHAT IS THE INTENDED USE?
The PanTum Detect® alone does not provide cancer diagnosis. It provides indications of elevated concentrations of DNaseX (Apo10) and TKTL1 in human EDTA whole blood. The qualitative test result identifies persons with increased glucose uptake and decreased apoptosis. Positive results can be associated with a possible tumor development, but do not rule out other underlying pathological changes. The test is intended to be used in the context of the preventive medical checkup. Persons with a positive result will be examined using suitable medical imaging techniques.
WHERE IS IT AVAILABLE?
🇩🇪 Germany
PanTum Detect® is currently available as part of the Krebs-Scan program by HanseMerkur:
KREBS-SCANONE TEST FOR ALMOST ALL?
In recent decades, scientists have extensively studied various approaches to improve the early detection of cancer. The most common approach has been to identify tumor-specific materials. This approach was justified by the assumption that different types of cancer have very little in common. However, this meant that each type of cancer required its own tumormarkers, making it an unlikely solution for the early detection of hundreds of different types of cancer.
Now this has changed. Thanks to the discovery of two genes, we are able to understand cancer on a much a deeper level: All types of cancer have two basic biophysical mechanisms in common:
- An impaired apoptosis (programmed cell death).
- A metabolic change, i.e. the way the cancer cells produce energy.
These findings finally paved the way for an early detection method which works for almost all types of cancer. The scientists behind PanTum Detect® use a new approach: Scanning the immune system for the two enzymes that identify two basic biophysical mechanisms. Indicators for cancer can thus be detected before it is too late.
The immune system continually combats cancer precursors. When cancer is diagnosed based on symptoms, it usually means that the immune system has been fighting it for many years already.
By analyzing and interpreting the remnants of these battles, PanTum Detect® is able to identify the appropriate moment to seek medical consultation for further diagnostics. Consequently, it has greatly facilitated the early detection of various types of cancer.
STRONG AND EXTENSIVE RESEARCH BASIS
Over the past 25 years, more than 60 scientific papers, publications and studies on TKTL1, DNaseX and EDIM technology, the essential components of the PanTum Detect®, have been produced and published in renowned journals such as Science and Nature Communications. These form the basis for the test development of the PanTum Detect® in its current form.
The PanTum Detect® has proven its performance in a large-scale prospective study as well as in several retrospective studies with diagnosed cancer patients.
The following is an excerpt of the performance data of the PanTum Detect® from some clinical studies.
STRONG AND EXTENSIVE RESEARCH BASIS
Over the past 25 years, more than 60 scientific papers, publications and studies on TKTL1, DNaseX and EDIM technology, the essential components of the PanTum Detect®, have been produced and published in renowned journals such as Science and Nature Communications. These form the basis for the test development of the PanTum Detect® in its current form.
The PanTum Detect® has proven its performance in a large-scale prospective study as well as in several retrospective studies with diagnosed cancer patients.
The following is an excerpt of the performance data of the PanTum Detect® from some clinical studies.
FIRST OVERVIEW OF A PROSPECTIVE CLINICAL STUDY*
Out of a group of >5,000 supposedly healthy persons, 186 tested PanTum Detect® positive. 151 of them underwent subsequent imaging procedures (MRI and/or PET/CT) which provided indications of a premalignant or malignant tumor for 124 persons and thereby confirmed the initial PanTum Detect® blood test results.
5,114
healthy, symptom-free individuals got tested with PanTum Detect®.
151
of the 186 subjects who tested PanTum Detect® positive underwent subsequent imaging procedures (MRI and/or PET/CT).
82%
In more than 82% of these cases (124 out of 151), subsequent imaging procedures provided indications of cancer or a precancerous tumor with a high risk of degeneration and thereby confirmed the initial PanTum Detect® results.
29
Within the study alone, indications for 29 different tumor entities – many of them in early and highly treatable stages – were provided with the combination of PanTum Detect® blood test and subsequent imaging (MRI and/or PET/CT).
Source: Burg et al. (2022)
*Study ongoing. Results are intermediary results. Final results expected throughout the course of 2024
RESULTS OF RETROSPECTIVE CLINICAL STUDIES
Throughout the last 10 years, 7 studies with diagnosed cancer patients have been performed at the University of Tübingen, Germany, to verify PanTum Detect®’s reliability to recognize sick people as sick (sensitivity).
The figures below represent a summary of the 7 studies.
652
diagnosed cancer patients have been tested with the PanTum Detect®.
96.2%**
was the overall sensitivity across these 7 studies with 627 out of 652 correctly positive PanTum Detect® results.
98.2%***
was the overall specificity across these 7 studies with only 3 out of 167 false-positive control group test results.
8
different types of cancer have been tested within these 7 studies with the PanTum Detect® sensitivity results significantly above 90% for all of them.
Source: Grimm et al (2013), Grimm et al (2016)1, Grimm et al (2016)2, Todenhöfer et al (2017), Saman et al (2020), Stagno et al (2022), Urla et al (2022)
** officially claimed sensitivity of 95.2% as per intended use
*** officially claimed specificity of 99.5% as per intended use


