Um die Chancen aller Betroffenen zu verbessern.
Zugang zum PanTum Detect® Portal (nur für Krebs-Scan Kunden):

EINFACH
Eine normale Blutentnahme einmal jährlich genügt.
FRÜHZEITIG
Erkennt Hinweise auf Tumoren bereits in frühen, symptomfreien Stadien.
WEGWEISEND
Grundlage für den gezielten Einsatz medizinischer Bildgebung (z.B. PET/CT, MRT).
EINER FÜR FAST ALLE
Liefert Hinweise auf fast alle Krebsarten.
BEDEUTUNG DER KREBSFRÜHERKENNUNG
In den meisten Krebsfällen wächst der Tumor über viele Jahre unentdeckt. Je früher er erkannt wird, desto besser sind die Chancen auf eine vollständige Heilung.
JEDER 2. MENSCH
ERKRANKT AN KREBS
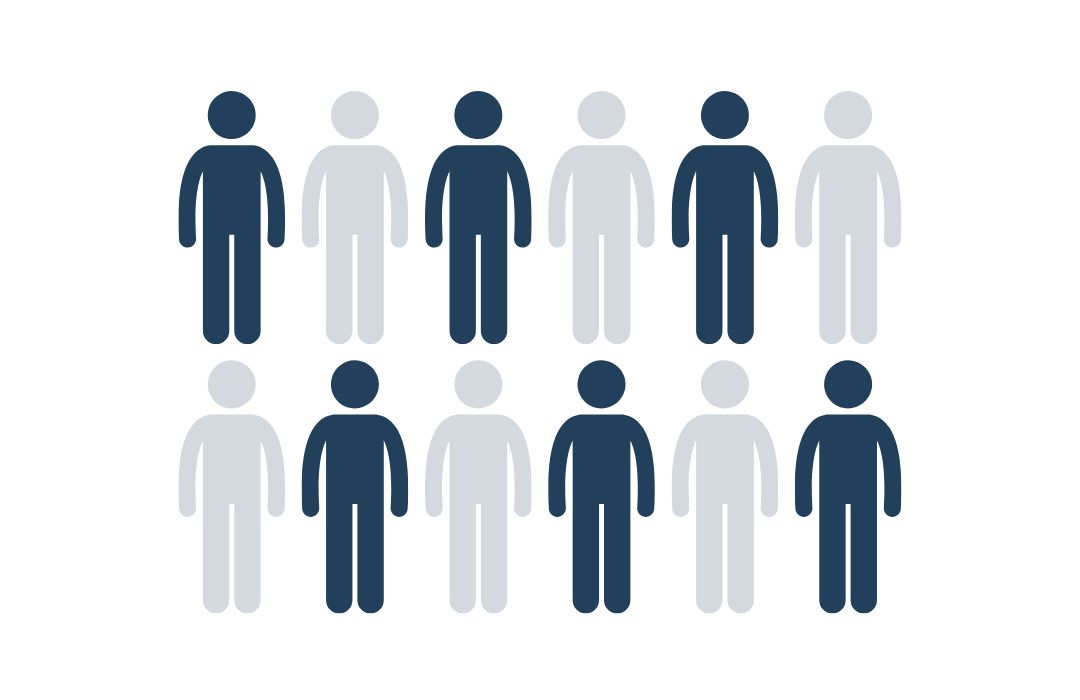
Statistisch gesehen erkrankt etwa jeder zweite Mensch einmal in seinem Leben an Krebs.
Quelle: Cancer Research UK
KREBSSPEZIFISCHE
VORSORGE
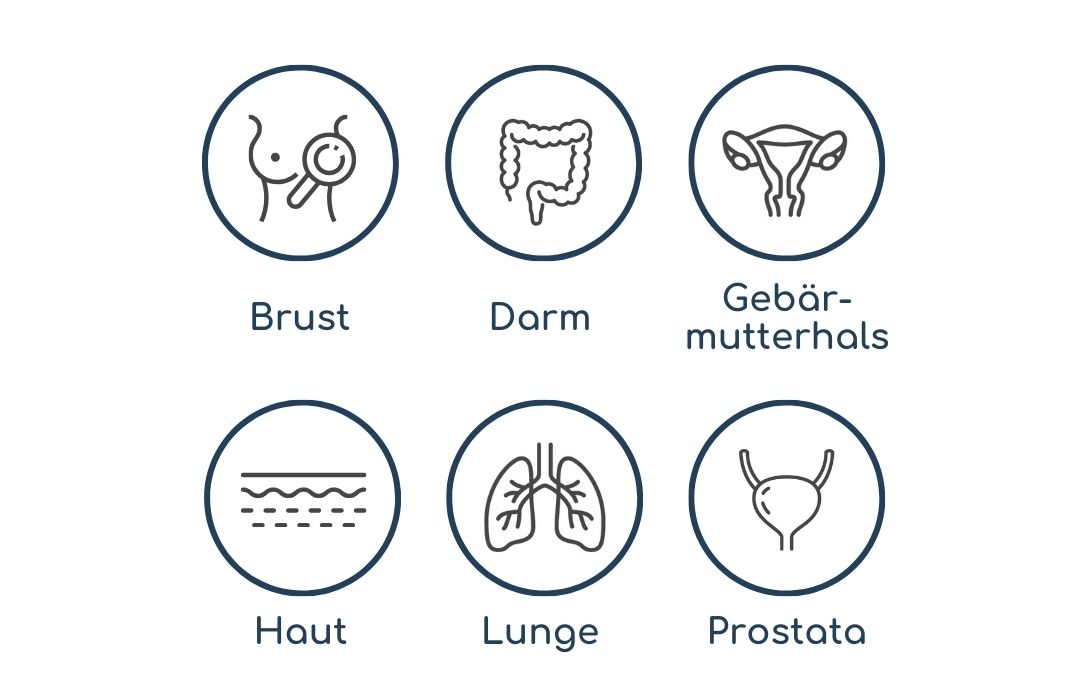
In ausgewählten Ländern gibt es bislang regelmäßige Krebs-Vorsorgeuntersuchungen für Brust-, Gebärmutterhals-, Darm-, Lungen-, Prostata- und Hautkrebs.
Quelle: Deutsches Krebsforschungszentrum (DKFZ); American Cancer Society
SCREENINGLÜCKE:
FALL DEUTSCHLAND
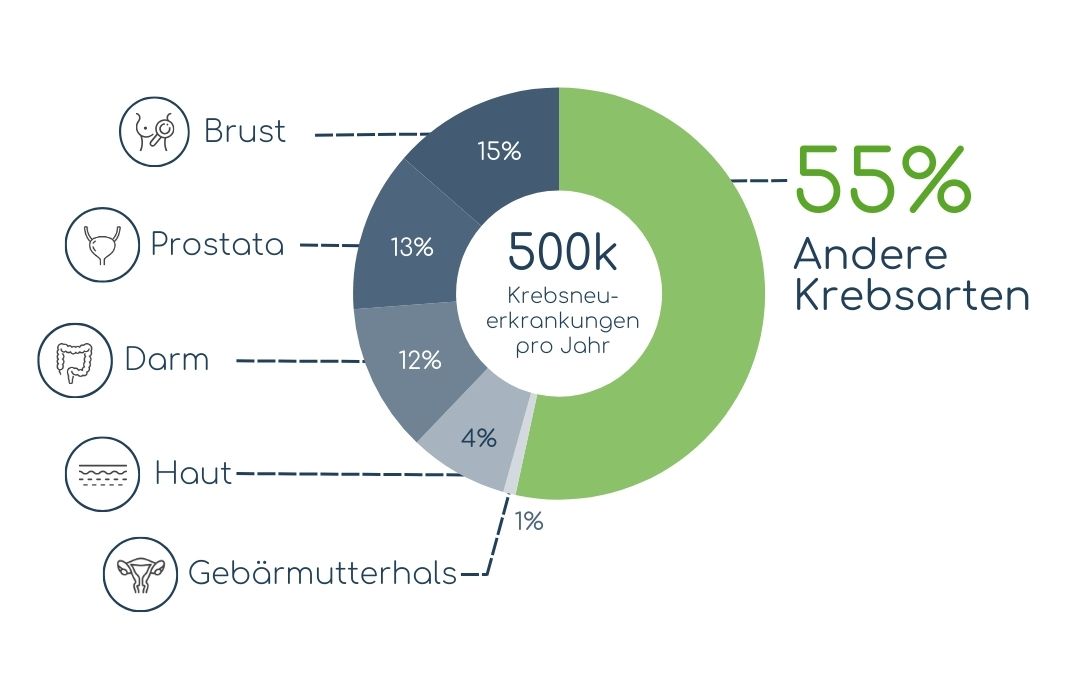
Selbst in einem medizinisch so fortgeschrittenen Land wie Deutschland gibt es für 55% der jährlich 500.000 Krebsneuerkrankungen keine regelmäßigen Vorsorgeuntersuchungen. Das entspricht mehr als 250.000 Fällen pro Jahr.
Quelle: Durchschnittliche jährliche Krebsneuerkrankungen basierend auf Daten des RKI (krebsdaten.de | 2009-2018)
PANTUM DETECT® – ENTWICKELT, UM ETWAS ZU BEWIRKEN
- Kann frühzeitig Hinweise auf potenzielle Tumoren liefern. Zu einem Zeitpunkt, an dem die Behandlungsmöglichkeiten vielfältig und die Heilungschancen hoch sind.
- Entwickelt, um rechtzeitig und im richtigen Stadium Hinweise zu erkennen – nicht zu früh, nicht zu spät.
- Nur eine Blutentnahme jährlich.
- Nicht spezifisch für eine Krebsart: Gilt für fast alle Krebsarten.
- Detektiert Signale für zwei biophysikalische Mechanismen, die allen Krebsarten gemeinsam sind.
- PanTum Detect® stellt keine Krebsdiagnose und identifiziert auch keine Krebsarten, sondern gibt allgemeine Hinweise auf eine mögliche Krebserkrankung, die in der Folgediagnostik, z. B. durch bildgebende Verfahren, bestätigt und lokalisiert werden müssen.
FÜR WEN IST DER PANTUM® DETECT?
Für all diejenigen, die …
Um zu beurteilen, ob eine Krebsfrüherkennungsuntersuchung für Sie in Ihrer persönlichen Situation (Alter, familiäre Vorbelastung, persönliche Krankheitsgeschichte, persönlicher Gesundheitszustand usw.) sinnvoll ist, wenden Sie sich bitte an Ihren Hausarzt.
WAS IST DER
VERWENDUNGSZWECK?
Der PanTum Detect® allein dient nicht der Krebsdiagnose. Er liefert Hinweise auf erhöhte Konzentrationen von DNaseX (Apo10) und TKTL1 in menschlichem EDTA-Vollblut. Das qualitative Testergebnis identifiziert Personen mit erhöhter Glukoseaufnahme und verringerter Apoptose. Positive Ergebnisse können mit einer möglichen Tumorentwicklung in Verbindung gebracht werden, schließen jedoch andere zugrunde liegende pathologische Veränderungen nicht aus. Der Test soll im Rahmen einer Vorsorgeuntersuchung eingesetzt werden. Personen mit einem positiven Ergebnis werden mit geeigneten medizinischen bildgebenden Verfahren weiter untersucht.
WO IST DER PANTUM DETECT® VERFÜGBAR?
Deutschland
Der PanTum Detect® ist aktuell als Teil des Krebs-Scan Programms der HanseMerkur Versicherungsgruppe verfügbar:
Indien
Der PanTum Detect® ist in Indien über unseren Partner Medidect kommerziell erhältlich:
EIN TEST FÜR FAST ALLE KREBSARTEN?
In den letzten Jahrzehnten haben Wissenschaftler intensiv verschiedene Ansätze zur Verbesserung der Krebsfrüherkennung untersucht. Der häufigste Ansatz war dabei die Identifizierung tumorspezifischer Besonderheiten. Dieser Ansatz war durch die Annahme gerechtfertigt, dass verschiedene Krebsarten nur sehr wenig gemeinsam haben. Dies bedeutete jedoch, dass jede Krebsart ihre eigenen Tumormarker benötigte, was eine Lösung für die Früherkennung von Hunderten verschiedener Krebsarten gleichzeitig unwahrscheinlich machte.
Dies hat sich nun geändert. Dank der Entdeckung zweier Gene sind wir nun in der Lage, Krebs auf einer viel tieferen Ebene zu verstehen. Alle Arten von Krebs haben zwei grundlegende biophysikalische Mechanismen gemeinsam:
- Eine gestörte Apoptose (programmierter Zelltod).
- Eine Veränderung des Stoffwechsels, in der Art und Weise, wie die Krebszellen Energie produzieren.
Diese Erkenntnisse ebneten schließlich den Weg für eine Früherkennungsmethode, die bei fast allen Krebsarten funktioniert. Die Wissenschaftler, die hinter PanTum Detect® stehen, verfolgen einen neuen Ansatz: Sie suchen im Immunsystem nach den beiden Enzymen, die die beiden grundlegenden biophysikalischen Mechanismen identifizieren. So können Anzeichen für Krebs erkannt werden, bevor es zu spät ist.
Das Immunsystem bekämpft kontinuierlich Krebsvorläufer. Wenn Krebs aufgrund von auftretenden Symptomen diagnostiziert wird, bedeutet dies in der Regel, dass das Immunsystem den Krebs bereits seit vielen Jahren bekämpft hat.
Durch die Analyse und Interpretation der Überreste dieser Kämpfe ist PanTum Detect® in der Lage, den richtigen Zeitpunkt für eine ärztliche Beratung zur weiteren Diagnostik zu ermitteln und hat so die Früherkennung verschiedener Krebsarten bereits erheblich erleichtert.
STARKE UND UMFASSENDE FORSCHUNGSGRUNDLAGE
In den vergangenen 25 Jahren wurden mehr als 60 wissenschaftliche Arbeiten, Publikationen und Studien zu TKTL1, DNaseX und der EDIM-Technologie, den wesentlichen Komponenten des PanTum Detect®, durchgeführt und in renommierten Fachjournalen wie Science und Nature Communications veröffentlicht. Auf dieser wissenschaftlichen Grundlage wurde der PanTum Detect® Test in seiner heutigen Form entwickelt.
Die Leistungsfähigkeit des PanTum Detect® wurde sowohl in einer groß angelegten prospektiven Studie als auch in mehreren retrospektiven Studien mit diagnostizierten Krebspatienten bewiesen.
Im Folgenden finden Sie einen Überblick der Leistungsdaten des PanTum Detect® aus einigen klinischen Studien.
ERSTER ÜBERBLICK EINER PROSPEKTIVEN
KLINISCHEN STUDIE*
Aus einer Gruppe von >5.000 vermeintlich gesunden Personen testete der PanTum Detect® 186 positiv. 151 von ihnen unterzogen sich anschließend bildgebenden Verfahren (MRT und/oder PET/CT), die bei 124 Personen Hinweise auf einen prämalignen oder malignen Tumor lieferten und damit die initialen PanTum Detect® Bluttestergebnisse bestätigten.
5.114
gesunde, symptomfreie Personen wurden mit dem PanTum Detect® getestet.
151
der 186 Probanden mit auffälligem PanTum Detect® Ergebnis ließen sich anschließend mittels bildgebender Verfahren (MRT und/oder PET/CT) weiter untersuchen.
82%
In mehr als 82 % dieser Fälle (124 von 151) lieferten die bildgebenden Folgeuntersuchungen Hinweise auf Krebs oder eine Krebsvorstufe mit hohem Entartungsrisiko und bestätigten damit die initialen PanTum Detect® Ergebnisse.
29
Allein im Rahmen der Studie wurden mit der Kombination aus PanTum Detect® Bluttest und anschließender Bildgebung (MRT und/oder PET/CT) Hinweise auf 29 verschiedene Tumorentitäten – viele davon in frühen und gut behandelbaren Stadien – geliefert.
Quelle: Burg et al. (2022)
*Studie noch nicht abgeschlossen. Die Ergebnisse sind Zwischenergebnisse. Endgültige Ergebnisse werden im weiteren Verlauf 2024 erwartet.
ERGEBNISSE RETROSPEKTIVER KLINISCHER STUDIEN
In den letzten 10 Jahren wurden an der Universität Tübingen 7 Studien mit diagnostizierten Krebspatienten durchgeführt, um zu prüfen, dass der PanTum Detect® kranke Menschen zuverlässig als krank erkennt (Sensitivität).
Die nachfolgenden Zahlen liefern eine Zusammenfassung der Ergebnisse dieser 7 Studien.
652
diagnostizierte Krebspatienten wurden mit dem PanTum Detect® getestet.®.
96,2%**
war die Gesamt-Sensitivität über alle 7 Studien hinweg mit 627 von 652 korrekt positiven PanTum Detect® Ergebnissen.
98,2%***
war die Gesamt-Spezifität über alle 7 Studien hinweg mit nur 3 von 167 falsch-positiven Testergebnissen in der Kontrollgruppe.
8
verschiedene Krebsarten wurden in diesen 7 Studien getestet, wobei die Sensitivität des PanTum Detect® bei allen getesteten Krebsarten deutlich über 90% lag.
Quelle: Grimm et al (2013), Grimm et al (2016)1, Grimm et al (2016)2, Todenhöfer et al (2017), Saman et al (2020), Stagno et al (2022), Urla et al (2022)
** offiziell angegebene Sensitivität gemäß Verwendungszweck: 95,2%
*** offiziell angegebene Spezifität gemäß Verwendungszweck: 99,5%
WAS SIND SOLIDE TUMOREN?
Krebs bedeutet, dass sich Zellen des eigenen Körpers verändern. Sie erfüllen nicht mehr ihre eigentliche Aufgabe, vermehren sich unkontrolliert und wachsen in gesundes Gewebe ein. Das kann dann dazu führen, dass Gewebe und Organe nicht mehr richtig funktionieren.
Viele Krebsformen wachsen zunächst als Geschwulst an einer bestimmten Stelle im Körper. Man spricht dabei von einem soliden Tumor.
Solide Tumoren machen etwa 90 % der Krebsneuerkrankungen bei Erwachsenen aus. Sie können in Knochen, Muskeln, Organen oder Bindegewebe auftreten. Sie unterscheiden sich von flüssigen Tumoren, bei denen die Krebszellen durch den Blutkreislauf oder das Lymphsystem zirkulieren, anstatt eine feste Masse zu bilden. Zu den flüssigen Tumoren zählen Leukämien (Blutkrebs) und einige Lymphome (Krebserkrankungen des lymphatischen Systems).
Der PanTum Detect® kann Hinweise auf solide, nicht aber auf flüssige Tumoren finden.


